What caused the ‘Space Shuttle Challenger Disaster'?
Posted on
Chemistry is a very important aspect in our day-to-day life. The significance of chemistry in industry, pharmacy, medicine, agriculture and different orbits of life is not unknown to all. It can be equally important and lifesaving in the field of aerospace also. Even once the world witnessed a tragic accident of NASA because of lack of some basic knowledge of chemistry. Let us go through the incident.
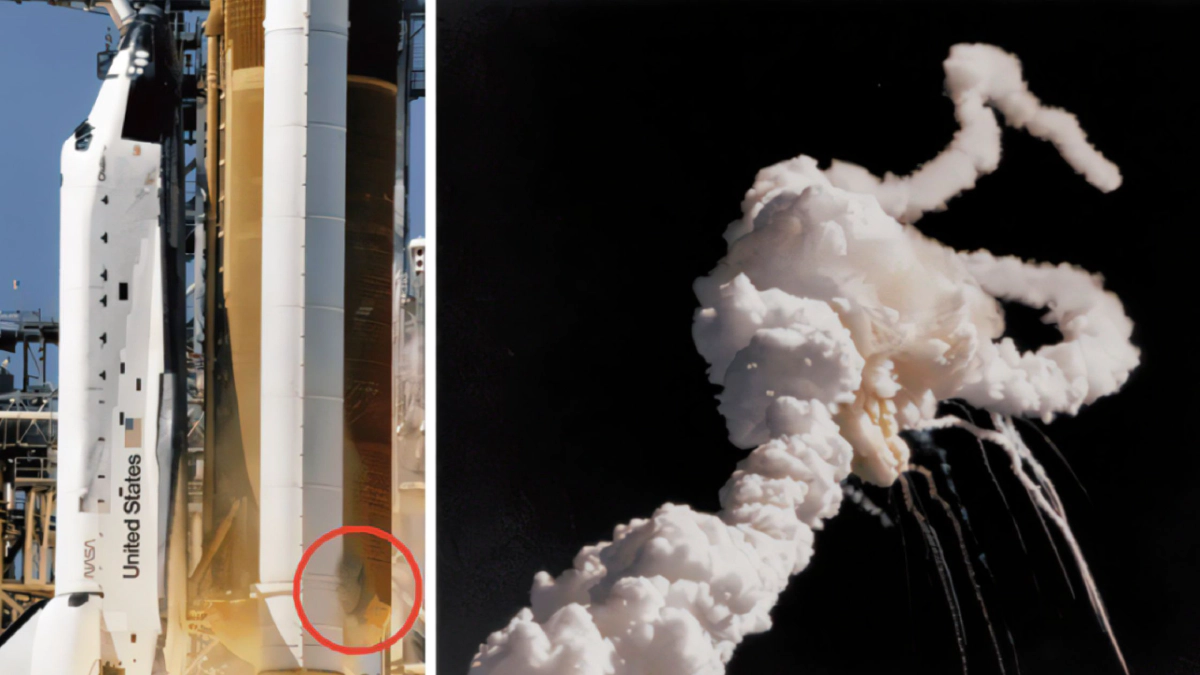
In 1986, ‘The Space Shuttle Challenger Disaster' of NASA made the whole world spellbound. This accident occurred during the 10th launch of Space Shuttle Challenger. Seven crew members died tragically after this fatal accident.
What was the reason for this accident?
This accident occurred because of the fundamental design flaws. Investigations show that the low temperature caused the hot gases and flames to leak out. The main reason for the leak was the failure of flexibility of a tiny rubber part ‘O-ring' which resulted in fuel escapement and caused explosion. Now, let us see why the ring failed. Actually, the failure of this ring is significantly related to chemistry. Glass transition temperature is a very important aspect of amorphous plastics and elastomers. Basically, it is defined as the temperature at which the transition from rigid state to more flexible state occurs. Though amorphous plastics work best below glass transition temperature, elastomers must be used above this border line. Below the glass transition temperature, elastomers act as glass and could be broken easily when bent. That’s why the O-ring failed in low temperature.
From this accident, the significance of elastomers being above glass transition temperature was clarified. Moreover, rectifying their previous mistakes NASA redesigned their next projects ensuring necessary safety. This incident depicts the importance of chemistry in every sphere of life and shows that even in the field of aerospace, the importance of chemistry is enormous.